Facts About Water
Yes, of course the most obvious fact about water is that it is wet, at least in the liquid state. But, there are many more facts about water that make it a most fascinating substance, one that all life on and in the Earth depends on.
• Water Science School HOME • Water Basics topics • Water Properties topics •
Water numbers
Some of water's physical properties:
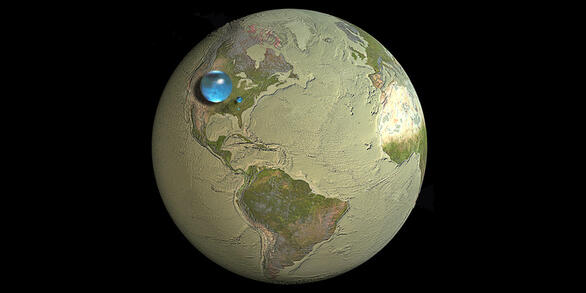
- Weight: 62.416 pounds/cubic foot at 32°F; 1,000 kilograms/cubic meter
- Weight: 61.998 pounds/cubic foot at 100°F; 993 kilograms/cubic meter
- Weight: 8.33 pounds/gallon; 1 kilogram/liter
- Density: 1 gram/cubic centimeter (cc) at 39.2°F, 0.95865 gram/cc at 212°F
Some water volume comparisons:
- 1 gallon = 4 quarts = 8 pints = 128 fluid ounces = 3.7854 liters
- 1 liter = 0.2642 gallons = 1.0568 quart
- 1 million gallons = 3.069 acre-feet = 133,685.64 cubic feet
Flow rates:
- 1 cubic foot/second (cfs) = 449 gallons/minute = 0.646 million gallons/day = 1.98 acre-feet/day
Water facts

Water is called the "universal solvent" because it dissolves more substances than any other liquid. This means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients.
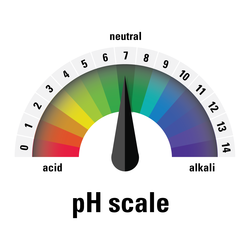
Pure water has a neutral pH of 7, which is neither acidic (less than 7) nor basic (greater than 7).
The water molecule is highly cohesive — it is very sticky, meaning water molecules stick to each other. Water is the most cohesive among the non-metallic liquids.
Water molecules are also adhesive in that they stick to other surfaces. Both cohesion and adhesion make water molecules very sticky!
Pure water, which you won't ever find in the natural environment, does not conduct electricity. Water becomes a conductor once it starts dissolving substances around it.
Water has a high heat index—it absorbs a lot of heat before it begins to get hot. This is why water is valuable to industries and in your car's radiator as a coolant. The high heat index of water also helps regulate the rate at which air changes temperature, which is why the temperature change between seasons is gradual rather than sudden, especially near the oceans.
Water has a very high surface tension. In other words, water is sticky and elastic, and tends to clump together in drops rather than spread out in a thin film, like rubbing alcohol. Surface tension is responsible for capillary action, which allows water (and its dissolved substances) to move through the roots of plants and through the tiny blood vessels in our bodies.
Air pressure affects the boiling point of water, which is why it takes longer to boil an egg at Denver, Colorado than at the beach. The higher the altitude, the lower the air pressure, the lower the boiling point of water, and thus, the longer time to hard-boil an egg. At sea level water boils at 212°F (100°C), while at 5,000 feet, water boils at 202.9°F (94.9 °C).
Other science topics related to water properties.
Water Properties Information by Topic
pH and Water
Water Properties Questions & Answers
Surface Tension and Water
Teacher's Resources for Water Education
The USGS Water Science School offers many resources to help teach students all about water.
Water Properties Photo Gallery
Water, the Universal Solvent
Conductivity (Electrical Conductance) and Water
Specific Heat Capacity and Water
Water Density
Capillary Action and Water
Adhesion and Cohesion of Water
Yes, of course the most obvious fact about water is that it is wet, at least in the liquid state. But, there are many more facts about water that make it a most fascinating substance, one that all life on and in the Earth depends on.
• Water Science School HOME • Water Basics topics • Water Properties topics •
Water numbers
Some of water's physical properties:
- Weight: 62.416 pounds/cubic foot at 32°F; 1,000 kilograms/cubic meter
- Weight: 61.998 pounds/cubic foot at 100°F; 993 kilograms/cubic meter
- Weight: 8.33 pounds/gallon; 1 kilogram/liter
- Density: 1 gram/cubic centimeter (cc) at 39.2°F, 0.95865 gram/cc at 212°F
Some water volume comparisons:
- 1 gallon = 4 quarts = 8 pints = 128 fluid ounces = 3.7854 liters
- 1 liter = 0.2642 gallons = 1.0568 quart
- 1 million gallons = 3.069 acre-feet = 133,685.64 cubic feet
Flow rates:
- 1 cubic foot/second (cfs) = 449 gallons/minute = 0.646 million gallons/day = 1.98 acre-feet/day
Water facts

Water is called the "universal solvent" because it dissolves more substances than any other liquid. This means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients.
Pure water has a neutral pH of 7, which is neither acidic (less than 7) nor basic (greater than 7).
The water molecule is highly cohesive — it is very sticky, meaning water molecules stick to each other. Water is the most cohesive among the non-metallic liquids.
Water molecules are also adhesive in that they stick to other surfaces. Both cohesion and adhesion make water molecules very sticky!
Pure water, which you won't ever find in the natural environment, does not conduct electricity. Water becomes a conductor once it starts dissolving substances around it.
Water has a high heat index—it absorbs a lot of heat before it begins to get hot. This is why water is valuable to industries and in your car's radiator as a coolant. The high heat index of water also helps regulate the rate at which air changes temperature, which is why the temperature change between seasons is gradual rather than sudden, especially near the oceans.
Water has a very high surface tension. In other words, water is sticky and elastic, and tends to clump together in drops rather than spread out in a thin film, like rubbing alcohol. Surface tension is responsible for capillary action, which allows water (and its dissolved substances) to move through the roots of plants and through the tiny blood vessels in our bodies.
Air pressure affects the boiling point of water, which is why it takes longer to boil an egg at Denver, Colorado than at the beach. The higher the altitude, the lower the air pressure, the lower the boiling point of water, and thus, the longer time to hard-boil an egg. At sea level water boils at 212°F (100°C), while at 5,000 feet, water boils at 202.9°F (94.9 °C).
Other science topics related to water properties.
Water Properties Information by Topic
pH and Water
Water Properties Questions & Answers
Surface Tension and Water
Teacher's Resources for Water Education
The USGS Water Science School offers many resources to help teach students all about water.